WELCOME TO HEAD & NECK ROBOTIC SURGERY IN THE H&N
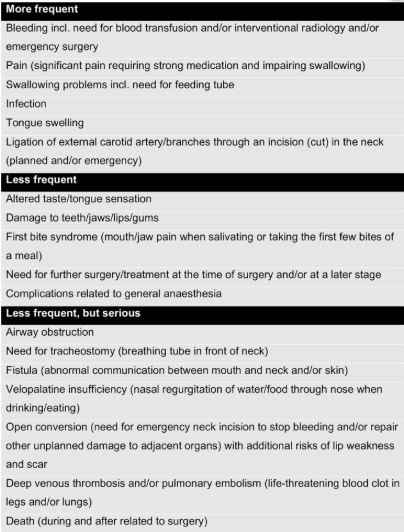
A group of UK colleagues has just made a proposal to be used in the informed consent framework for Transoral Robotic Surgery (TORS). They developed the document using a Delphi expert consensus methodology (1). Their work resulted in a recommended list of complications to be included in patient consent forms for TORS procedures.
Those are:
For the past 20 years, every surgeon performing TORS has likely used a specific informed consent document. In our case, Dr. Raimundo Gutiérrez Fonseca and I drafted our own version back in 2012, prior to treating our first TORS patient. We based it on existing templates for other transoral procedures, adapting it to the robotic platform.
We submitted our document to the Commission of Protocols and Standards of the Spanish Society of Otolaryngology – Head and Neck Surgery (SEORL-CCC), which formally adopted it. Since then, it has served as the official national protocol in Spain. As part of our international training activity we have also provided the document, both in Spanish and in English, to colleagues from other countries worldwide (see bellow).
A written informed consent is a legal requirement for any invasive medical procedure. Beyond oral explanations provided by the physician, these cases require a signed document to ensure patient autonomy and legal protection. Some elements are compulsory, such as:
Over time, our approach to TORS has evolved (like that of every surgeon who has practised though the development of the technique). We were all understandably cautious in the early days—for instance, staging the primary surgery and the neck dissection, or routinely using nasogastric feeding tubes. But today, TORS has become a routine and well-established procedure for various indications.
Perhaps now is the right moment to update our informed consent document, incorporating real-world data and current standards of care.
J Granell. May 18, 2025