WELCOME TO HEAD & NECK ROBOTIC SURGERY IN THE H&N
Transforming patient care through precision, minimally invasive techniques, and condition-specific surgical solutions.
It appears to be clear that most of the surgical procedures that now we perform with conventional endoscopic instruments will migrate to robotization in a short period of time.
Some years ago the reasons to start a robotic surgical program were the technological positioning in a competitive market and to offer cutting edge surgery. Today, although still dealing with efficiency and optimization of outcomes, the expansion of minimally invasive surgery by robotic surgery programs is already a fact.
So it is more a matter of not being left behind and taking advantage of the known and the unpredictable possibilities.
Organization in paramount. The robotic surgery program is an investment of strategic magnitude. Adequate management is paramount.
Who is in the Robotic Surgery Committee? It might vary among Hospitals but there are three basic components.
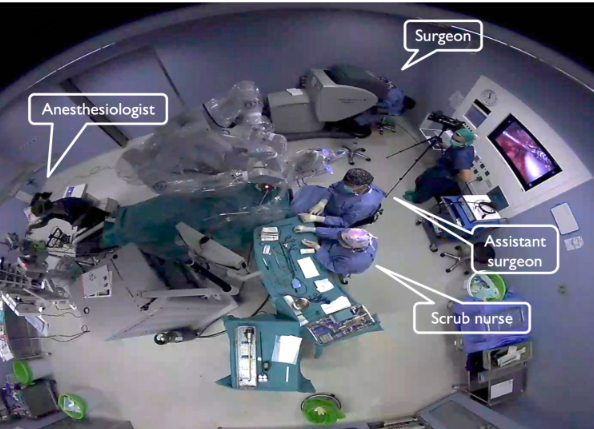
Robotic surgery introduces a fundamental shift in the dynamics of the operating room (OR) compared to conventional surgery. In traditional procedures, the surgeon stands directly at the operating table, working hands-on with instruments and in close proximity to the patient. In contrast, robotic-assisted surgery positions the surgeon at a console, often several feet away from the surgical field. From this console, the surgeon telemanipulates robotic arms equipped with precision instruments, translating their hand movements into fine, tremor-free actions within the patient. This separation requires not only a rethinking of the physical space but also adjustments in how the surgical team communicates and collaborates during the procedure.
Additionally, since these robotic ORs are often shared by multiple specialties—including ENT, urology, gynecology, and general surgery—they must be designed with flexibility in mind. Modular layouts, mobile equipment, and adaptable workflows are essential to accommodate the diverse requirements of different surgical disciplines, ensuring efficient turnover and optimal use of the robotic platform.
Also every robotic platform has peculiarities. Let’s examine the details of the set-up for every robotic device, categorized into two distinct groups of surgical techniques in robotic HNS: TransOral Robotic Surgery (TORS) and Robotic Remote Access techniques in H&N Surgery.
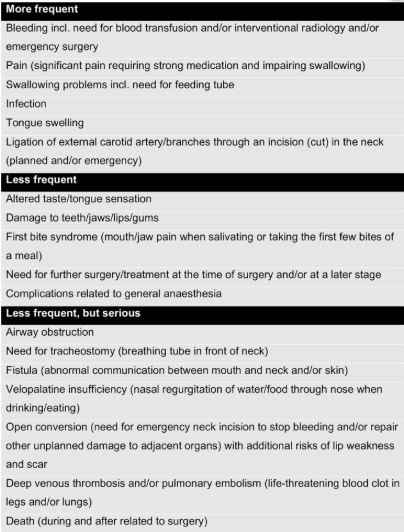
A group of UK colleagues has just made a proposal to be used in the informed consent framework for Transoral Robotic Surgery (TORS). They developed the document using a Delphi expert consensus methodology (1). Their work resulted in a recommended list of complications to be included in patient consent forms for TORS procedures.
Those are:
For the past 20 years, every surgeon performing TORS has likely used a specific informed consent document. In our case, Dr. Raimundo Gutiérrez Fonseca and I drafted our own version back in 2012, prior to treating our first TORS patient. We based it on existing templates for other transoral procedures, adapting it to the robotic platform.
We submitted our document to the Commission of Protocols and Standards of the Spanish Society of Otolaryngology – Head and Neck Surgery (SEORL-CCC), which formally adopted it. Since then, it has served as the official national protocol in Spain. As part of our international training activity we have also provided the document, both in Spanish and in English, to colleagues from other countries worldwide (see bellow).
A written informed consent is a legal requirement for any invasive medical procedure. Beyond oral explanations provided by the physician, these cases require a signed document to ensure patient autonomy and legal protection. Some elements are compulsory, such as:
Over time, our approach to TORS has evolved (like that of every surgeon who has practised though the development of the technique). We were all understandably cautious in the early days—for instance, staging the primary surgery and the neck dissection, or routinely using nasogastric feeding tubes. But today, TORS has become a routine and well-established procedure for various indications.
Perhaps now is the right moment to update our informed consent document, incorporating real-world data and current standards of care.
J Granell. May 18, 2025
References
(1) Gupta KK, Simons A, Mortimore S, Walker D, Sahota RB, De M, Arora A, Garas G. Consensus regarding transoral robotic surgery (TORS) complications for use in a proposed standardised consent form. J Robot Surg. 2025 May 15;19(1):217. doi: 10.1007/s11701-025-02389-z. PMID: 40372502